What is NAD+ and Why Is it Important for Aging and Health?
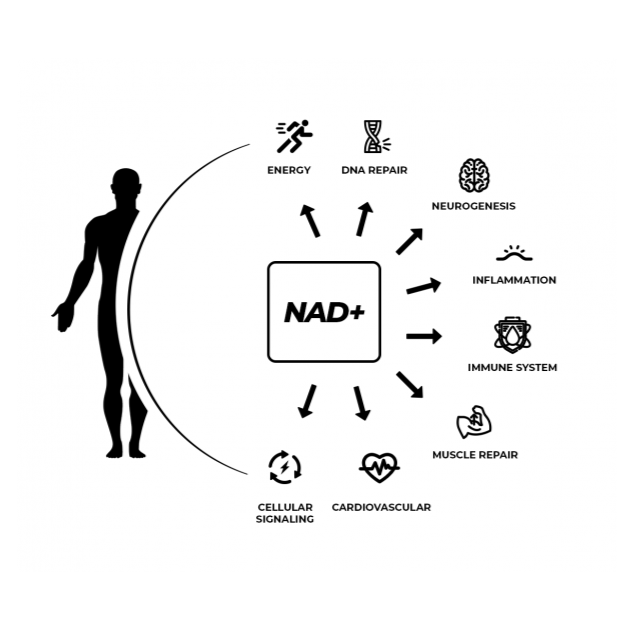
Open any biology textbook and you’ll learn about NAD+, which stands for nicotinamide adenine dinucleotide. It’s a critical coenzyme found in every cell in your body that’s involved in hundreds of metabolic processes like cellular energy and mitochondrial health. NAD+ is hard at work in the cells of humans and other mammals, yeast and bacteria, even plants.
Scientists have known about NAD+ since it was first discovered in 1906, and since then our understanding of its importance has continued to evolve. For example, the NAD+ precursor niacin played a role in mitigating pellagra, a fatal disease that plagued the American south in the 1900s. Scientists at the time identified that milk and yeast, which both contain NAD+ precursors, alleviated symptoms. Over time scientists have identified several NAD+ precursors — including nicotinic acid, nicotinamide, nicotinamide riboside ( NR) and nicotinamide mononucleotide ( NMN ), among others — which make use of natural pathways that lead to NAD+. Think of NAD+ precursors as different routes you can take to get to a destination. All the pathways get you to the same place but by different modes of transportation.
Recently, NAD+ has become a prized molecule in scientific research because of its central role in biological functions. The scientific community has been researching how NAD+ relates to notable benefits in animals that continue to inspire researchers to translate these findings to humans. So how exactly does NAD+ play such an important role? In short, it’s a coenzyme or “helper” molecule, binding to other enzymes to help cause reactions on the molecular level.
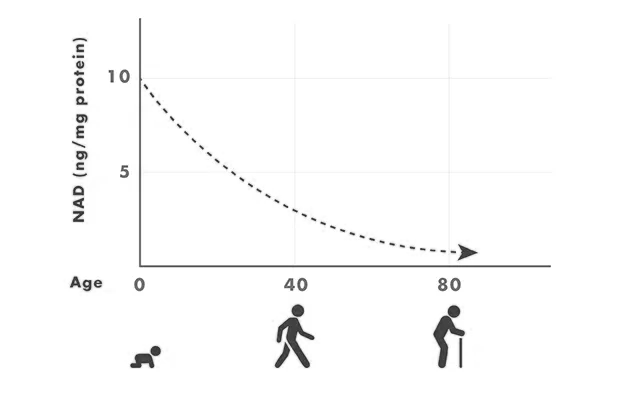
But the body doesn’t have an endless supply of NAD+. In fact, it actually declines with age.
The history of NAD+ research, and its recent establishment in the science community, has opened the floodgates for scientists to investigate maintaining NAD+ levels and getting more NAD+.
Scientists have known about NAD+ since it was first discovered in 1906, and since then our understanding of its importance has continued to evolve. For example, the NAD+ precursor niacin played a role in mitigating pellagra, a fatal disease that plagued the American south in the 1900s. Scientists at the time identified that milk and yeast, which both contain NAD+ precursors, alleviated symptoms. Over time scientists have identified several NAD+ precursors — including nicotinic acid, nicotinamide, nicotinamide riboside ( NR) and nicotinamide mononucleotide ( NMN ), among others — which make use of natural pathways that lead to NAD+. Think of NAD+ precursors as different routes you can take to get to a destination. All the pathways get you to the same place but by different modes of transportation.
Recently, NAD+ has become a prized molecule in scientific research because of its central role in biological functions. The scientific community has been researching how NAD+ relates to notable benefits in animals that continue to inspire researchers to translate these findings to humans. So how exactly does NAD+ play such an important role? In short, it’s a coenzyme or “helper” molecule, binding to other enzymes to help cause reactions on the molecular level.
But the body doesn’t have an endless supply of NAD+. In fact, it actually declines with age.
The history of NAD+ research, and its recent establishment in the science community, has opened the floodgates for scientists to investigate maintaining NAD+ levels and getting more NAD+.
The Importance of NAD+
Our current understanding of the importance of NAD+ really began in the 1960s. Using nuclear extracts from hen liver, French scientist Pierre Chambon identified a process called Poly ADP-ribosylation, where NAD+ is broken down into two component parts, one of which (nicotinamide) gets recycled, while the other (ADP-ribose) meets up with a protein. This research formed the foundation of the field of PARPs, or poly (ADP-ribose) polymerases, a group of proteins that rely on NAD+ to function and perform cellular functions. PARPs are similar to another group of proteins called sirtuins in that they both only function in the presence of NAD+.
Scientists often refer to sirtuins as “guardians of the genome” for their role in regulating cellular homeostasis. Homeostasis involves keeping the cell in balance. Sirtuins are a group of proteins that were first discovered in the 1970s but their dependence on NAD+ wasn’t realized until the 1990s. MIT biologist Leonard Guarente identified that SIR2, a sirtuin in yeast, extended the life of the yeast only when it was activated by NAD+.
Knowing this created a clear link between sirtuins and metabolism. It also clued scientists in on a crosstalk between biological functions, i.e., that metabolism is intricately related to other biological processes. Additionally, it inspired more research on a topic previously overlooked.
Humans get NAD+ from their diet via foods made up of amino acids that are also precursors to NAD+. However, NR and NMN are a highly efficient precursors to NAD+. If NAD+ precursors are different routes you can take to get to a destination, NMN is often thought to be the best available route to NAD+.
Scientists often refer to sirtuins as “guardians of the genome” for their role in regulating cellular homeostasis. Homeostasis involves keeping the cell in balance. Sirtuins are a group of proteins that were first discovered in the 1970s but their dependence on NAD+ wasn’t realized until the 1990s. MIT biologist Leonard Guarente identified that SIR2, a sirtuin in yeast, extended the life of the yeast only when it was activated by NAD+.
Knowing this created a clear link between sirtuins and metabolism. It also clued scientists in on a crosstalk between biological functions, i.e., that metabolism is intricately related to other biological processes. Additionally, it inspired more research on a topic previously overlooked.
Humans get NAD+ from their diet via foods made up of amino acids that are also precursors to NAD+. However, NR and NMN are a highly efficient precursors to NAD+. If NAD+ precursors are different routes you can take to get to a destination, NMN is often thought to be the best available route to NAD+.
How Sirtuins Regulate Cellular Health with NAD+
Think of your body’s cells like an office. In the office, there are many people working on various tasks with an ultimate goal: stay profitable and fulfill the mission of the company in an efficient manner for as long as possible. In the cells, there are many pieces working on various tasks with an ultimate goal, too: stay healthy and function efficiently for as long as possible. Just as priorities in the company change, due to various internal and external factors, so do priorities in the cells. Someone has to run the office, regulating what gets done when, who’s going to do it and when to switch course. In the office, that would be your CEO. In the body, at the cellular level, it’s your sirtuins.
Sirtuins are a family of seven proteins that play a role in cellular health. Sirtuins can only function in the presence of NAD+, nicotinamide adenine dinucleotide, a coenzyme found in all living cells. NAD+ is vital to cellular metabolism and hundreds of other biological processes. If sirtuins are a company’s CEO, then NAD+ is the money that pays the salary of the CEO and employees, all while keeping the lights on and the office space rent paid. A company, and the body, can’t function without it. But levels of NAD+ decline with age, limiting the function of sirtuins with age as well.
Sirtuins Are Proteins. What Does That Mean?
Sirtuins are a family of proteins. Protein might sound like dietary protein — what’s found in beans and meats and well, protein shakes — but in this case we’re talking about molecules called proteins, which work throughout the body’s cells in a number of different functions. Think of proteins as the departments at a company, each one focusing on its own specific function while coordinating with other departments.
A well-known protein in the body is hemoglobin, which is part of the globin family of proteins and is responsible for transporting oxygen throughout your blood. The myoglobin is the hemoglobin’s counterpart, and together they make up the globin family.
Your body has nearly 60,000 families of proteins — a lot of departments! — and sirtuins are one of those families. While hemoglobin is one in a family of two proteins, sirtuins are a family of seven.
Of the seven sirtuins in the cell, three of them work in the mitochondria, three of them work in the nucleus and one of them works in the cytoplasm, each playing a variety of roles. The basic role of sirtuins, however, is that they remove acetyl groups from other proteins.
Sirtuins work with acetyl groups by doing what’s called deacetylation. This means they recognize there’s an acetyl group on a molecule then remove the acetyl group, which tees up the molecule for its job. One way that sirtuins work is by removing acetyl groups (deacetylating) biological proteins such as histones. For example, sirtuins deacetylate histones, proteins that are part of a condensed form of DNA called chromatin. The histone is a large bulky protein that the DNA wraps itself around. Think of it as a Christmas tree, and the DNA strand is the strand of lights. When the histones have an acetyl group, the chromatin is open, or unwound.
This unwound chromatin means the DNA is being transcribed, an essential process. But it doesn’t need to remain unwound, as it’s vulnerable to damage in this position, almost like the Christmas lights could get tangled or the bulbs can get damaged when they’re unwieldy or up for too long. When the histones are deacetylated by sirtuins, the chromatin is closed, or tightly and neatly wound, meaning gene expression is stopped, or silenced.
We’ve only known about sirtuins for about 20 years, and their primary function was discovered in the 1990s. Since then, researchers have flocked to study them, identifying their importance while also raising questions about what else we can learn about them.